Due to a greater understanding of the health and environmental impacts related to the disposal and release of PFAS into the environment, interest in finding viable methods for removing PFAS from the environment is growing.
By Viraj deSilva, PhD, PE, BCEE
Perfluoroalkyl and polyfluoroalkyl substances (PFAS) are a collection of fluorine-containing organic compounds that have been used and produced in the U.S. since the early 1940s. These compounds are popular due to their moisture, oil and grease resistance attributes. The water repellant properties make them popular for use in textiles (raincoats, umbrellas), paper products (pizza boxes, popcorn bags, burger and sandwich wrappers), leather (water resistant boots), and materials like waxes, paint and adhesives. PFAS are also used in firefighting foams for extinguishing flammable liquid fires.
Due to a greater understanding of the health and environmental impacts related to the disposal and release of PFAS into the environment, interest in finding viable methods for removing PFAS from the environment is growing. According to the Agency for Toxic Substances and Disease Registry (ATSDR), PFAS have been linked to serious adverse health effects, including increased cholesterol levels, interference with the body’s natural hormones, decreased fertility, thyroid and liver diseases, immune system diseases and cancer.
In addition to the presence of PFAS in consumer and household products, studies show that exposure to PFAS can occur through potable water, food packaging, stormwater runoff, PFAS-containing wastes and leachate from landfills. Even though the oldest PFAS compounds are no longer manufactured, they remain in our environment and in the human body for a prolonged period of time.
The EPA and scientific communities across the country have renewed their focus on per- and poly-fluoroalkyl substances (primarily PFOA and PFOS) collectively known as PFAS. The EPA’s PFAS health advisory limit is 70 parts per trillion (PPT). Currently, a growing number of states throughout the U.S. are dealing with PFAS in the water environment.
PFAS Chemistry and Properties
PFAS are generally carbon chains of varying length and can include varying amounts of oxygen, hydrogen and fluorine. PFAS compounds are highly persistent, mobile, water soluble and toxic. Some PFAS are so persistent that they are hard to degrade in the environment, and, therefore, the PFAS levels will only get higher over time if their use continues.
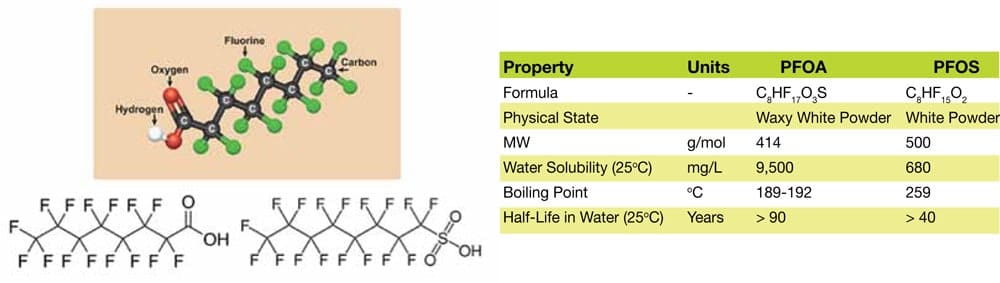
PFOA are composed of a fully-fluorinated backbone with an alcohol functional group. PFOS has an eight-carbon, fully-fluorinated backbone with an added sulfonate functional group. The chemical structures of PFAS compounds are shown in Figure 1, and their properties are listed in Table 1.
PFAS Sources
PFAS do not occur naturally in the environment. They are manufactured chemicals and are widely used in non-stick cookware, stain resistant carpets and fabrics, waterproof mattresses and clothes, and used to make some food packaging resistant to grease absorption, such as fast-food wrappers, and microwave popcorn bags. PFOS are also used in some firefighting materials. Figure 2, shows a few PFAS-containing products.

Human Exposure to PFAS
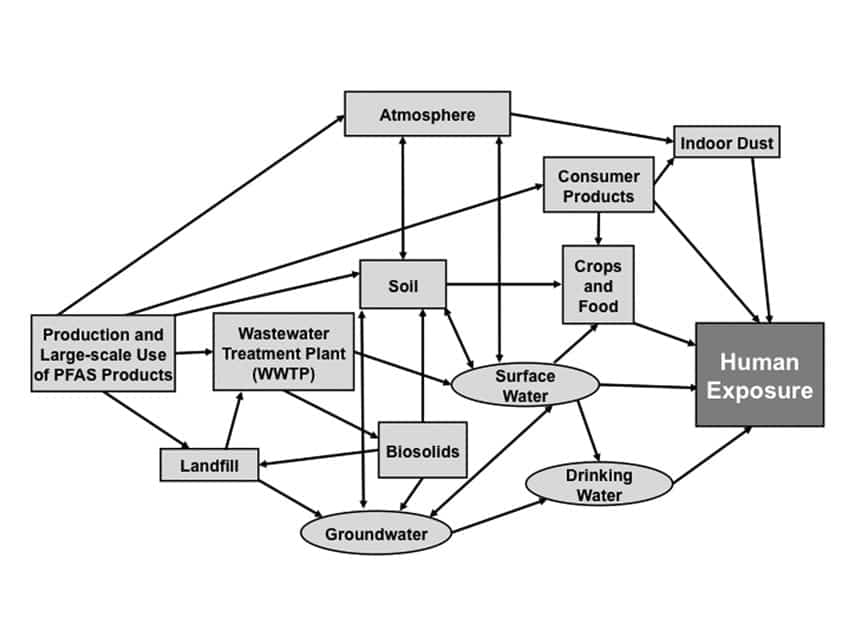
PFAS are found in all indoor and outdoor environments across the globe (Blum et al. 2015). The range of PFAS properties allows some to migrate through groundwater and surface water (rivers, streams, and lakes), be released into the atmosphere, returned in precipitation and adsorbed by soil. Some PFAS are bioaccumulated into food crops, livestock, wildlife, and the tissues and bodily fluids of humans through consumption of contaminated foods and drinking water, and direct contact with various consumer products (Figure 3). Each transport process has the potential for differential fractionation of individual PFAS, including bioaccumulation, which elevates levels in the surrounding environment.
People are exposed to PFAS chemicals not only during normal use of PFAS-containing products, but also during biodegradation and disposal of consumer products. People who work at PFAS production facilities or facilities that manufacture goods made with PFAS may be exposed through contaminated air or other means.
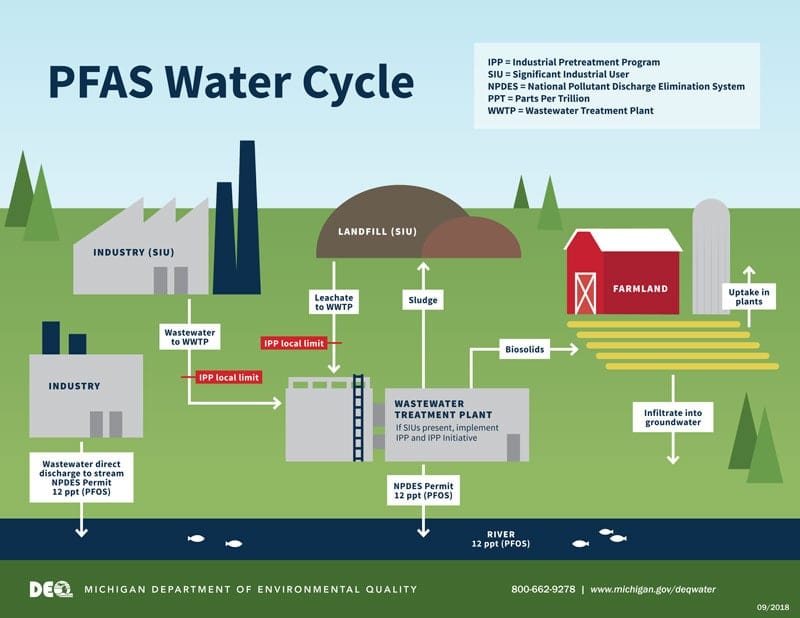
PFAS Water Cycle
Drinking water can be a source of exposure in communities where PFAS chemicals have contaminated the water supplies. Such contamination is typically localized and is associated with an industrial facility that manufactures products that contain PFAS. The wastewater treatment plant then further disperses the PFAS by discharging them to a surface water body that pollutes the drinking water source. PFAS chemicals are extremely persistent in the environment, and environmental contamination continues long after the active contamination has stopped. A number of states have initiated procedures to reduce and eliminate certain PFAS compounds from industrial sources that may pass through municipal wastewater treatment plants (WWTP) and pollute public drinking water supplies. For example, landfill leachates containing PFAS chemicals are being rejected at WWTPs. Figure 4, page 47, published by the Michigan Department of Environmental Quality, diagrams the PFAS water cycle.
Regulation of PFAS
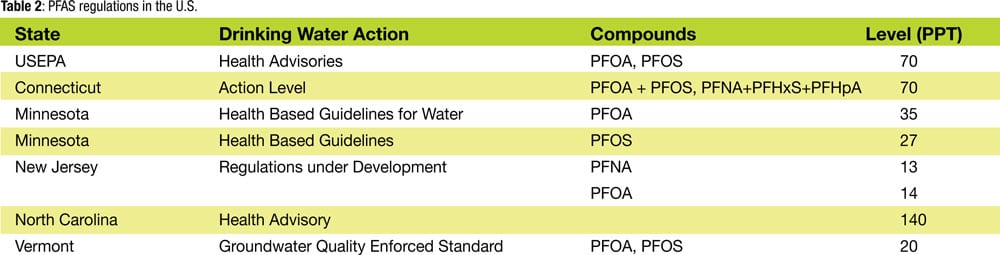
Drinking water guidelines in the U.S. vary from state to state, and no federal maximum contaminant level (MCL) has been established for PFAS chemicals in drinking water. However, in June 2016, the U.S. EPA established a combined public health advisory level for PFOA and PFOS of 70 parts per trillion in drinking water. Some states have taken a more stringent approach to PFAS in their drinking water. Table 2 presents a summary of how the U.S. EPA and several states are handling the various PFAS chemicals that have effects similar to those of PFOS and PFOA.
Treatment of PFAS
Public water systems may be able to reduce PFAS chemical concentrations by closing contaminated wells or by blending water sources if allowed. Treatment processes that can remove PFAS chemicals from drinking water may include activated carbon, ion exchange or high-pressure membrane systems. The more conventional water treatment technologies such as (e.g., aeration) are not typically effective.
Granular Activated Carbon
Activated carbon is a demonstrated technology and is currently the most commonly used treatment technology for PFAS removal. Removal efficiencies of between 90 percent and > 99 percent have been reported in the literature. The lower values likely are due to the inefficient removal of the shorter chain PFAS (Interstate Technology Regulatory Council (ITRC), 2018). GAC is available as virgin or reactivated material, both of which exhibit similar removal efficiencies from 90 to 99 percent. According to the ITRC, re-agglomerated bituminous coal provides the best performance. Longer chain PFAS and sulfonates are more readily adsorbed than shorter chain PFAS and carboxylates. Using carbon technologies for removal requires regeneration or replacement and disposal.
Ion Exchange/Anionic Exchange
Ion exchange, or anionic exchange, is a demonstrated technology that uses synthetic polymeric media, which are set up similarly to, and can be combined with, GAC. Ion exchange uses positively charged media to remove negatively charged PFAS molecules.
A combination of technologies may be the best approach in order to overcome the limitations of ionic exchange. The National Groundwater Association (NGWA) cites removal efforts by a New Jersey WWTP that used anionic exchange, but found that shorter chain PFAS were not removed (NGWA Groundwater and PFAS: Section 8, Remediation and Treatment). Similarly, other studies suggest that GAC does not address a growing list of PFAS compounds. A combination of adsorption followed by anionic exchange would remove both the longer and shorter chain PFAS.
Membrane Filtration
Membrane filtration is described as a “salt passage” and “salt rejection” system. This technology has been used at WWTP for decades. Salt combines with certain compounds and increases their molecular weight; they are then too heavy to pass through the membrane and are thus separated from the water. The separation is based on the molecular weight cut-off (MWCO) of the membrane compared to the effective diameter of the molecules. Based on the MWCO for PFAS, microfiltration (MF) and ultrafiltration (UF) are unsuitable for PFAS treatment; however, reverse osmosis (RO) and nanofiltration (NF) are viable options with documented efficiencies greater than 90 percent. The waste stream for reverse osmosis will contain salts, and membrane filters and filtrate will require disposal.
Reverse Osmosis
Reverse osmosis and nanofiltration systems have been shown to be effective for the removal of many types of molecules and ions. With reverse osmosis, PFAS are retained in the reject stream on the pressurized side of the membrane, which must be further treated to prevent the release of the PFAS back into the environment. Reverse osmosis has been shown to be effective at the flowrates typical in community water systems (ITRC, 2018); however, reverse osmosis is costly, and responsible treatment and disposal of the PFAS-enriched reject stream is necessary.
Nanofiltration is less expensive than reverse osmosis because it operates at lower pressures; however, it is still at a developmental stage and has not been used in pilot or full-scale operations (ITRC, 2018). PFOS removal efficiencies of 93 to 99 percent have been reported for both reverse osmosis and nanofiltration membranes (Speth et al. 2018). Dickenson and Higgins (2016) reported removals greater than 90 percent for PFOA and PFOS and several other PFAS, including PFPeA, PFHxA, PFHpA, PFNA, PFDA, PFBS and PFHxS.
Summary
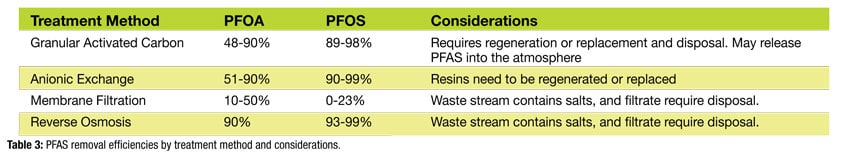
Disposal methods for PFAS waste streams include high temperature incineration or landfilling. Landfilling is not favored since the PFAS load would increase, and many landfills will not accept PFAS waste. Table 3 shows the PFOA and PFOS removal efficiencies of several treatment methods and/or other considerations. These treatment technologies have been lab or bench tested, field pilot tested, and are full-scale or commercially available.
Because of the widely varying properties of PFAS (e.g., persistence, water solubility, polarity, volatility), no single treatment method can remove all PFAS. Anionic Exchange and Granular Activated Carbon show promise for the removal of PFAS from drinking waters. While Reverse Osmosis has significant potential, just as with Anionic Exchange and GAC, it is more effective at removing longer-chain PFAS chemicals than short-chain compounds. However, laboratory-scale and pilot-scale studies are recommended before implementation since removal efficacy varies significantly depending on the types of PFAS present. In the case of Anionic Exchange and Reverse Osmosis, concentrated liquid waste streams must be further treated before they are discharged. With Granular Activated Carbon technologies, carbon regeneration has the potential to release PFAS into the atmosphere.
Anionic Exchange, Granular Activated Carbon and Reverse Osmosis can also be used to remove PFAS from wastewater effluent and landfill leachate. However, compared to most drinking waters, the presence of organic matter, inorganic chemicals, and particulates in wastewater and landfill leachate reduces their removal efficacy. For private drinking water supplies, certified point-of-use filters are commercially available to remove PFOA and PFOS.
All of the current water treatment technologies involve adsorption of PFAS into a support matrix, which then needs to be disposed or regenerated. High temperature incineration has been used for the oxidation of PFAS from solid material. None of the current technologies are capable of both removing and destroying PFAS simultaneously. Therefore, developing more effective and sustainable remediation solutions is vital.
Viraj deSilva, PhD, PE, BCEE is the Wastewater Treatment Director at SCS Engineers (Long Beach, CA). Dr. deSilva has conducted projects throughout the U.S. as well as in Japan, France, Australia, Ukraine, Venezuela, Guam, India, Sri Lanka, Korea, Qatar, Jordan, Kuwait, UAE and Iraq. He is experienced in the evaluation, sizing, and design of treatment processes for water/wastewater/leachate/solids handling facilities during his 29 years of experience in the field. He can be reached at (813) 804-6729, via e-mail at [email protected] or visit www.scsengineers.com/services/liquids-management/.
